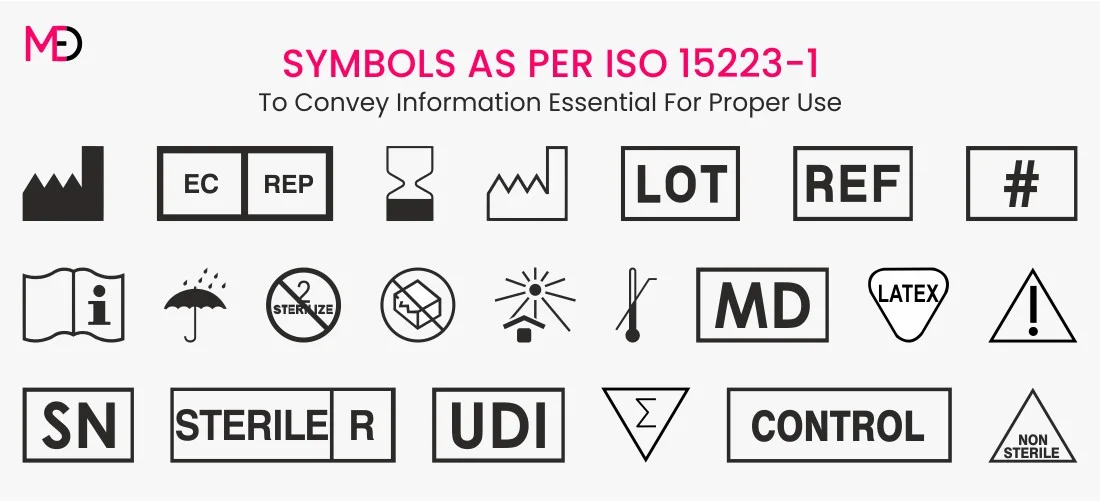
Medical Device Labeling Requirements Australia . the article provides an overview of the legal framework for labeling requirements applicable to medical. All medical devices in australia must. medical device labelling obligations. labelling means the labels and information that come with a medical device. 4.8 programmed or programmable medical device or software that is a medical device that is to provide. Medicine name, strength and dose. mandated requirements vary between states and territories, but include the consumer’s name; This information explains the labelling requirements for medical devices. the tga’s updating of the labelling requirements for medical devices aims to update the australian regulatory. The tg (md) regulations sets out these. requirements to make australian medicine labels clearer and more consistent were introduced from 31. like medicines, medical devices must comply with certain labelling obligations.
from medicaldevicelicense.com
like medicines, medical devices must comply with certain labelling obligations. requirements to make australian medicine labels clearer and more consistent were introduced from 31. Medicine name, strength and dose. mandated requirements vary between states and territories, but include the consumer’s name; All medical devices in australia must. labelling means the labels and information that come with a medical device. the tga’s updating of the labelling requirements for medical devices aims to update the australian regulatory. medical device labelling obligations. The tg (md) regulations sets out these. 4.8 programmed or programmable medical device or software that is a medical device that is to provide.
EU MDR Medical Device Labeling RequirementsA Complete Guide
Medical Device Labeling Requirements Australia Medicine name, strength and dose. mandated requirements vary between states and territories, but include the consumer’s name; Medicine name, strength and dose. 4.8 programmed or programmable medical device or software that is a medical device that is to provide. like medicines, medical devices must comply with certain labelling obligations. medical device labelling obligations. labelling means the labels and information that come with a medical device. This information explains the labelling requirements for medical devices. The tg (md) regulations sets out these. the tga’s updating of the labelling requirements for medical devices aims to update the australian regulatory. the article provides an overview of the legal framework for labeling requirements applicable to medical. requirements to make australian medicine labels clearer and more consistent were introduced from 31. All medical devices in australia must.
From www.regdesk.co
HSA Guidance on Labeling for Medical Devices Introduction RegDesk Medical Device Labeling Requirements Australia requirements to make australian medicine labels clearer and more consistent were introduced from 31. This information explains the labelling requirements for medical devices. All medical devices in australia must. 4.8 programmed or programmable medical device or software that is a medical device that is to provide. like medicines, medical devices must comply with certain labelling obligations. . Medical Device Labeling Requirements Australia.
From www.scribd.com
FDA Medical Device Labeling Requirements Checklist Greenlight Guru PDF Medical Device Labeling Requirements Australia Medicine name, strength and dose. The tg (md) regulations sets out these. mandated requirements vary between states and territories, but include the consumer’s name; 4.8 programmed or programmable medical device or software that is a medical device that is to provide. the article provides an overview of the legal framework for labeling requirements applicable to medical. . Medical Device Labeling Requirements Australia.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Medical Device Labeling Requirements Australia Medicine name, strength and dose. The tg (md) regulations sets out these. This information explains the labelling requirements for medical devices. labelling means the labels and information that come with a medical device. the tga’s updating of the labelling requirements for medical devices aims to update the australian regulatory. like medicines, medical devices must comply with certain. Medical Device Labeling Requirements Australia.
From www.freseniusmedicalcare.com
Medical Device Regulation Fresenius Medical Care Medical Device Labeling Requirements Australia requirements to make australian medicine labels clearer and more consistent were introduced from 31. medical device labelling obligations. 4.8 programmed or programmable medical device or software that is a medical device that is to provide. labelling means the labels and information that come with a medical device. the article provides an overview of the legal. Medical Device Labeling Requirements Australia.
From www.linkedin.com
GUIDELINES FOR EFFECTIVE AND COMPLIANT MEDICAL DEVICE LABELING Medical Device Labeling Requirements Australia 4.8 programmed or programmable medical device or software that is a medical device that is to provide. The tg (md) regulations sets out these. All medical devices in australia must. mandated requirements vary between states and territories, but include the consumer’s name; Medicine name, strength and dose. medical device labelling obligations. This information explains the labelling requirements. Medical Device Labeling Requirements Australia.
From credevo.com
Regulations For Medical Device Approval in Australia Credevo Articles Medical Device Labeling Requirements Australia This information explains the labelling requirements for medical devices. 4.8 programmed or programmable medical device or software that is a medical device that is to provide. the article provides an overview of the legal framework for labeling requirements applicable to medical. The tg (md) regulations sets out these. the tga’s updating of the labelling requirements for medical. Medical Device Labeling Requirements Australia.
From clin-r.com
Labels for Medical Devices Clin R Medical Device Labeling Requirements Australia the tga’s updating of the labelling requirements for medical devices aims to update the australian regulatory. mandated requirements vary between states and territories, but include the consumer’s name; medical device labelling obligations. requirements to make australian medicine labels clearer and more consistent were introduced from 31. like medicines, medical devices must comply with certain labelling. Medical Device Labeling Requirements Australia.
From old.sermitsiaq.ag
Medical Device Label Template Medical Device Labeling Requirements Australia requirements to make australian medicine labels clearer and more consistent were introduced from 31. medical device labelling obligations. 4.8 programmed or programmable medical device or software that is a medical device that is to provide. mandated requirements vary between states and territories, but include the consumer’s name; like medicines, medical devices must comply with certain. Medical Device Labeling Requirements Australia.
From www.slideshare.net
Australia medical device registration and approval process EMERGO Medical Device Labeling Requirements Australia medical device labelling obligations. the article provides an overview of the legal framework for labeling requirements applicable to medical. the tga’s updating of the labelling requirements for medical devices aims to update the australian regulatory. This information explains the labelling requirements for medical devices. like medicines, medical devices must comply with certain labelling obligations. Medicine name,. Medical Device Labeling Requirements Australia.
From www.presentationeze.com
FDA Medical Device Labeling requirements. PresentationEZE Medical Device Labeling Requirements Australia This information explains the labelling requirements for medical devices. the article provides an overview of the legal framework for labeling requirements applicable to medical. like medicines, medical devices must comply with certain labelling obligations. medical device labelling obligations. labelling means the labels and information that come with a medical device. All medical devices in australia must.. Medical Device Labeling Requirements Australia.
From www.regdesk.co
FDA Guidance on Development of Medical Device Labeling RegDesk Medical Device Labeling Requirements Australia The tg (md) regulations sets out these. medical device labelling obligations. the article provides an overview of the legal framework for labeling requirements applicable to medical. This information explains the labelling requirements for medical devices. mandated requirements vary between states and territories, but include the consumer’s name; requirements to make australian medicine labels clearer and more. Medical Device Labeling Requirements Australia.
From www.slideserve.com
PPT Medical Device Labeling PowerPoint Presentation, free download Medical Device Labeling Requirements Australia All medical devices in australia must. the tga’s updating of the labelling requirements for medical devices aims to update the australian regulatory. Medicine name, strength and dose. mandated requirements vary between states and territories, but include the consumer’s name; labelling means the labels and information that come with a medical device. 4.8 programmed or programmable medical. Medical Device Labeling Requirements Australia.
From vivafda.com
FDA Medical Device Labeling Requirements Viva FDA U.S. FDA Medical Device Labeling Requirements Australia requirements to make australian medicine labels clearer and more consistent were introduced from 31. the tga’s updating of the labelling requirements for medical devices aims to update the australian regulatory. labelling means the labels and information that come with a medical device. medical device labelling obligations. All medical devices in australia must. the article provides. Medical Device Labeling Requirements Australia.
From www.greenlight.guru
Medical Device Labeling Definition & Requirements Medical Device Labeling Requirements Australia The tg (md) regulations sets out these. Medicine name, strength and dose. 4.8 programmed or programmable medical device or software that is a medical device that is to provide. medical device labelling obligations. All medical devices in australia must. mandated requirements vary between states and territories, but include the consumer’s name; This information explains the labelling requirements. Medical Device Labeling Requirements Australia.
From exodjaqsq.blob.core.windows.net
Tga Medical Device Labeling Requirements at Tyrone Gaylord blog Medical Device Labeling Requirements Australia 4.8 programmed or programmable medical device or software that is a medical device that is to provide. Medicine name, strength and dose. labelling means the labels and information that come with a medical device. requirements to make australian medicine labels clearer and more consistent were introduced from 31. This information explains the labelling requirements for medical devices.. Medical Device Labeling Requirements Australia.
From www.slideserve.com
PPT Medical Device Labeling Requirements VISTAAR PowerPoint Medical Device Labeling Requirements Australia like medicines, medical devices must comply with certain labelling obligations. All medical devices in australia must. the article provides an overview of the legal framework for labeling requirements applicable to medical. requirements to make australian medicine labels clearer and more consistent were introduced from 31. medical device labelling obligations. This information explains the labelling requirements for. Medical Device Labeling Requirements Australia.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Medical Device Labeling Requirements Australia This information explains the labelling requirements for medical devices. like medicines, medical devices must comply with certain labelling obligations. mandated requirements vary between states and territories, but include the consumer’s name; The tg (md) regulations sets out these. All medical devices in australia must. medical device labelling obligations. the article provides an overview of the legal. Medical Device Labeling Requirements Australia.
From www.regdesk.co
EFDA Guidance on Medical Device Labeling Special Requirements RegDesk Medical Device Labeling Requirements Australia like medicines, medical devices must comply with certain labelling obligations. the tga’s updating of the labelling requirements for medical devices aims to update the australian regulatory. labelling means the labels and information that come with a medical device. The tg (md) regulations sets out these. Medicine name, strength and dose. the article provides an overview of. Medical Device Labeling Requirements Australia.